Discovery
Pre-clinical
Characterization
Clinical trial
Regulatory approval
Manufacturing
Product Launch
Postmarketing
surveillance
There is the need for complementary tools capable of restoring intestinal balance in the long term in patients with digestive diseases.
The prebiotic of GoodGut, with beneficial properties for patients with IBD and IBS, is a non-digestible fiber which has been developed to induce the selective growth of the protective bacteria. This increases their immunoprotective activity, such as the production of volatile fatty acids. In addition, it provides several advantages compared to probiotics:
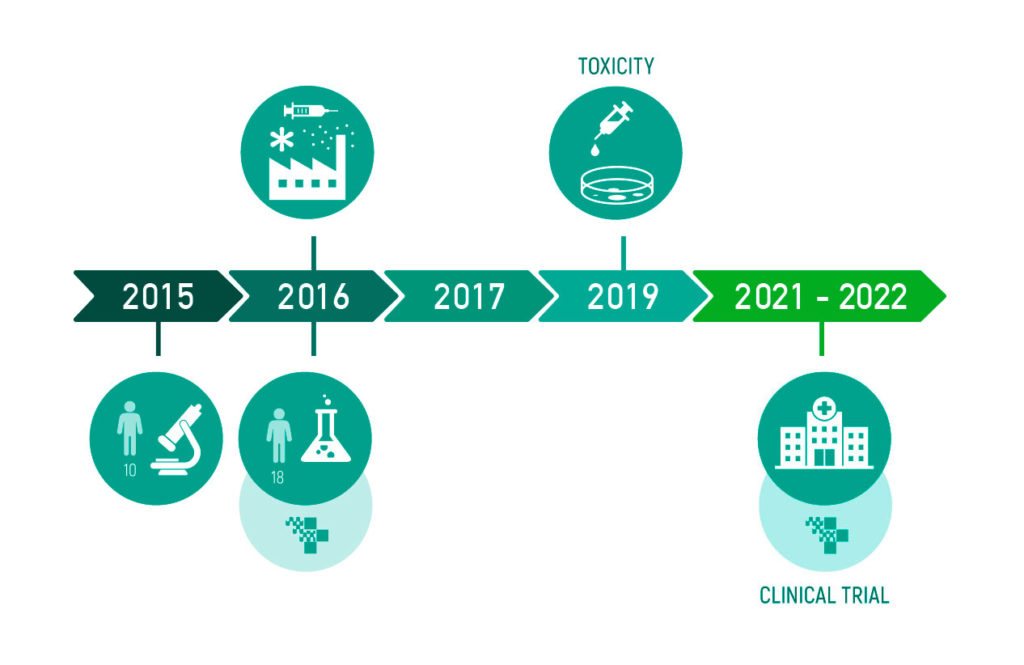
PREVIPECT has been assessed in a pilot study with fecal samples from 18 patients with IBD, IBS, and health subjects. The study consisted of evaluating the effect of the prebiotic on the fecal microbiota. It was carried out in collaboration with Hospital Universitari de Girona Dr. Josep Trueta. In addition, we completed theIn Vitrotoxicity test. For more information, you can check the results published in the study here(Oliver L. et al, 2021).
In order to validate the results observed in the pilot study, and demonstrate the benefits and added value of PREVIPECT in subjects with intestinal diseases such as IBD or IBS, the next step is to conduct clinical trials with the different target populations. With this, we plan to launch the product to the market in 2022.
The information contained on this page is intended exclusively for healthcare and/or laboratory professionals with the ability to diagnose digestive diseases and/or analyse the microbiota, which a specialized training is required for its correct interpretation. If you are not a qualified healthcare and/or laboratory professional, GoodGut is not responsible for any prejudice you may suffer from decisions based on the content of this page or any of its links. The information provided to goodgut.eu serves to support, not replace, the relationship that exists between a patient/visitor of this website and their physician.