
Discovery
Pre-clinical
Characterization
Clinical trial
Regulatory approval
Manufacturing
Product Launch
Postmarketing
surveillance

450 subjects with CRC-compatible symptomatology
172 FIT + subjects from a screening population

327 FIT + subjects from a screening population
2.800 FIT +/- subjects from a screening population
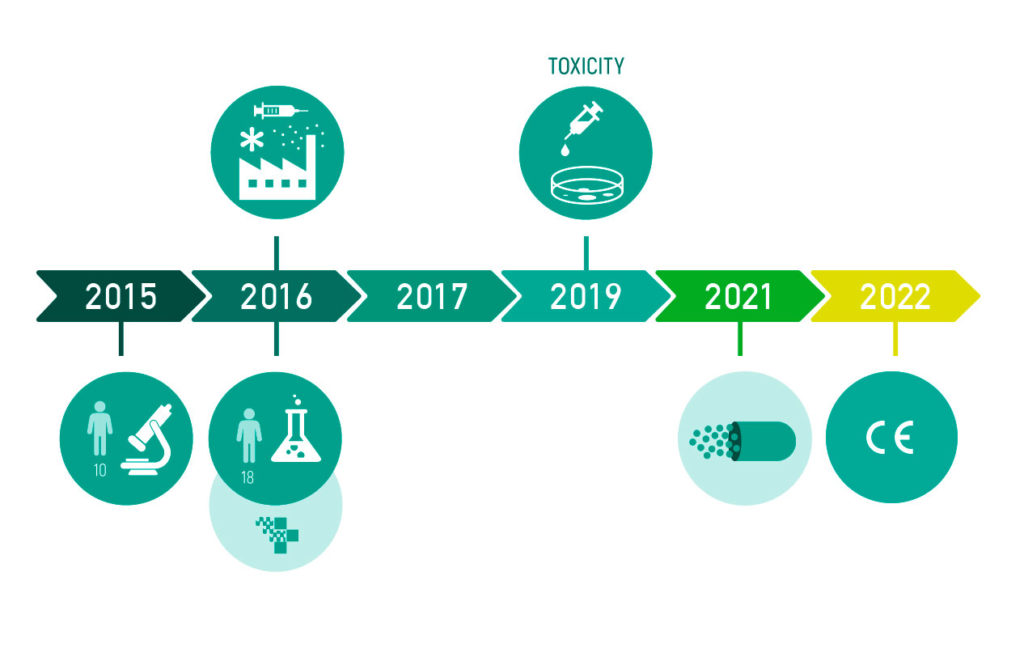
PREVIPECT has been tested in a pilot study with fecal samples from 18 patients with Inflammatory Bowel Disease, Irritable Bowel Syndrome, and health subjects, studying the effect of the prebiotic on the fecal microbiota, in collaboration with Hospital Universitari de Girona Dr. Josep Trueta. In addition, we completed theIn Vitro toxicity test. For more information, you can check the results published in the study here (Oliver L. et al, 2021).
In order to be able to administer the optimal amount to provide the greatest benefit, encapsulation assays will be carried out. With this, we plan to register the product in 2022.

The information contained on this page is intended exclusively for healthcare and/or laboratory professionals with the ability to diagnose digestive diseases and/or analyse the microbiota, which a specialized training is required for its correct interpretation. If you are not a qualified healthcare and/or laboratory professional, GoodGut is not responsible for any prejudice you may suffer from decisions based on the content of this page or any of its links. The information provided to goodgut.eu serves to support, not replace, the relationship that exists between a patient/visitor of this website and their physician.